Introduction
Several types of additives can be used in the dispersion process in which solid particles, like pigments and fillers, are distributed and stabilised in a liquid.
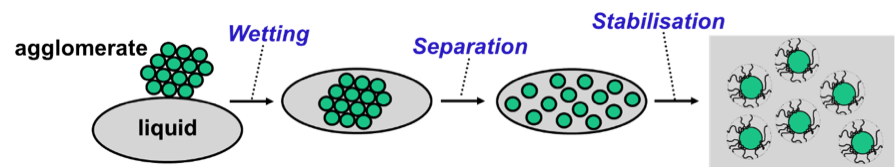
Often two categories of additives, wetting agent (EU) and dispersants (EU), are mentioned in one breath. However, the two materials differ strongly with respect to the role they play in the system and with respect to chemical composition and morphology of the molecules they are composed of.
Functionality
It is important to have a clear view on what each raw material that is used in a paint or ink should do. The job a raw material, like an additive, must do in a system is called functionality. It describes in a few words what the system developer would like the additive to do. A check question that can be asked in case you are not sure about the functionality of a component: What will happen when I remove the additive from the formulation?
Wetting agents
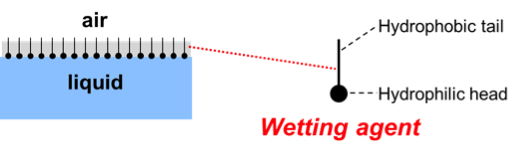
Wetting is the first step in the dispersion process. The air that surrounds the solid particles in an agglomerate must be substituted by liquid. Wetting will take place when the surface tension of the liquid is low compared to the surface energy of the solid particles. Wetting will not occur when the surface tension of the liquid is too high. In that case, the surface tension of the liquid can be lowered by adding a wetting agent. A wetting agent does its job because the molecules adsorb and orient on the liquid-air interface.
Dispersants
Solid particles attract each other. For this reason, energy is needed to separate the particles from each other in the second step of the dispersion process. Also, solid particles must be stabilised after they have been separated from each other. The particles will move to each other and glue together again when particle-particle repulsion is insufficient. The spontaneous process of gluing together of solid particles in a liquid is called flocculation. The functionality of a dispersant is to prevent flocculation. Dispersants do their job because the molecules adsorb on the solid-liquid interface and assure repulsion between the particles.
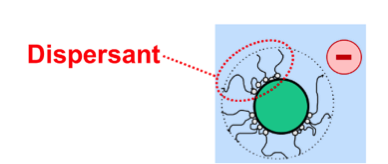
Repulsion can result from two mechanisms that may either be used separately or in combination:
- Electrostatic stabilisation: all particles carry a charge of the same sign.
- Steric stabilisation: all particles are covered with tails dissolving in the liquid that surrounds the particles.
Overview
Wetting agents and dispersants are additives that are used in dispersion processes. Both are interface additives but there are fundamental differences between the two types of raw materials.

System developers want to predict the behaviour of the additives they use. The behaviour of wetting agents and dispersants depends upon their chemical composition and molecular morphology. Useful information can be obtained by studying the documentation of the additives, like TDS (technical data sheets) and SDS (safety data sheets). The surface composition of the solid particles and the composition of the continuous liquid phase must also be known in order to be able to select the right additives.
Find our TDS on our Wetting Agent’s Product Page: Wetting Agent (Water Based) | Wetting Agent (Solvent Based)




Leave a Reply