
Historically, the Iron Oxides market has grown at a 2%-4% per year growth rate. There have been cyclical ups and downs but over a long term, this rate holds true. Its current use is mostly in construction, coatings, ceramics, paint, ink, rubber, plastics, cosmetics etc.
Usage in different industries:
The building materials industry is the biggest user of Iron Oxide Pigments. Uses include colouring concrete and mortar because of their good dispersability and good tinting strength. They are extensively used in the manufacture of Paving Blocks, Chequered Tiles, Designer Tiles, Stamped Concrete etc.
Paints industry is the second largest user of Iron Oxide Pigments. Most paint applications require use if micronized and dispersible grades of pigments to get optimum colouring effects and maintain paint film strength.
Pigments used in Plastics need high purity, tinting strength, high heat resistance and good dispersibility.
Special applications of Synthetic red Iron oxide include that in Ceramic Colours, Drugs, Cosmetics, Rubber, Brake Linings, Wood Polish, Cork Sheets, Fertilizers and Cattle Feed. New applications include catalysts in petroleum industry, in oil drilling rigs, in compound pigments, copy machine powder, and magnetic differentiate materials.
Manufacturing Process of Synthetic red Iron oxide:
A number of different processes have been developed for manufacturing Synthetic Iron Oxide Pigments. The most important actually used production methods are-
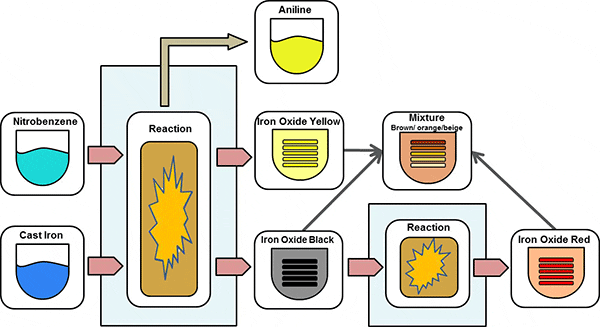
- LAUX PROCESSIn the Laux Process Iron Oxides are obtained as a by-product of the aniline process. The main pigment obtained is Black Iron Oxide, partially also Yellow Iron Oxide. These pigments are sold directly or used for preparation of Red Iron Oxide in the Calcination Process.
- CALCINATION PROCESSThe Calcination Process uses Black Iron Oxide to oxidize it to Red or Yellow Iron Oxide to dehydrate it to red, too. High temperatures are required for this process. A precise control of the process parameters also allows the production of brown pigments.
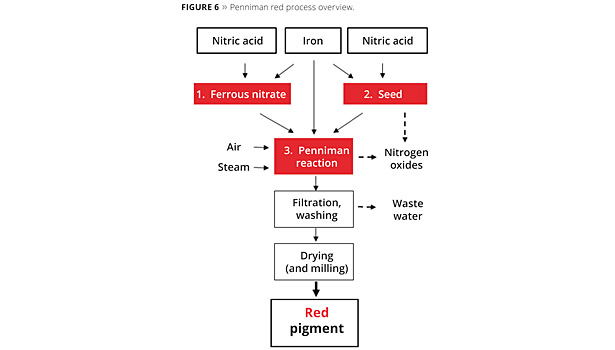
- PENNIMAN PROCESSThe Penniman Process reacts metallic iron to Iron Oxide using oxygen from air and acids as catalysts. Recently the process has been developed further to also give high quality red and black pigments.
- PRECIPITATION PROCESSIn the Precipitation Process iron salts react with caustic soda in the presence of air or oxygen. This process is controlled by temperature, pH, purity, concentration, mix design and reaction velocity. The several parameters influencing the product makes the precipitation process technically the most complicated but also produces pigments with an extremely high quality.
You can view the TDS of Synthetic Red Iron oxide offered by Esaar International Pvt. Ltd.: Click Here





Leave a Reply